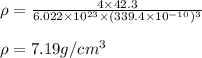Chemistry, 22.08.2019 08:30 domenica19
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell that is face-centered cubic. calculate the density of metal x? (atomic weight = 42.3 g/mol)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 05:30, victoria6929
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell...
Questions in other subjects:

Mathematics, 15.06.2021 17:10

Mathematics, 15.06.2021 17:10


English, 15.06.2021 17:10

Mathematics, 15.06.2021 17:10

Mathematics, 15.06.2021 17:10


English, 15.06.2021 17:10


Biology, 15.06.2021 17:10

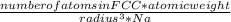 . Substituting the given, the density is 162.69 g/cm3.
. Substituting the given, the density is 162.69 g/cm3. 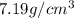
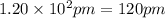
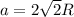
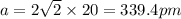
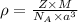
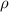 = density
= density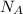 = Avogadro's number =
= Avogadro's number = 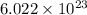
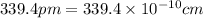 (Conversion factor:
(Conversion factor: 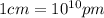 )
)