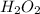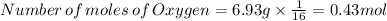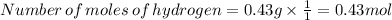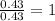Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

You know the right answer?
You find that 7.36g of a compound has decomposed to give 6.93g of oxygen. the only other element is...
Questions in other subjects:

Mathematics, 24.11.2020 21:40


World Languages, 24.11.2020 21:40

English, 24.11.2020 21:40

Mathematics, 24.11.2020 21:40

Biology, 24.11.2020 21:40