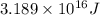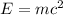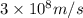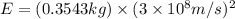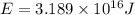Chemistry, 08.12.2019 00:31 llamasking
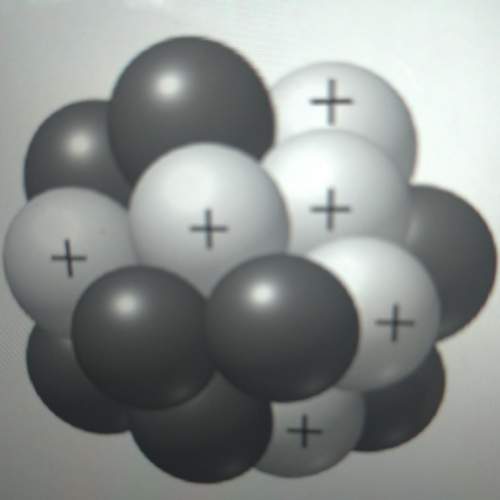
50 points! the isotope shown has a mass of 14.003241 amu. calculate how much energy is released from the binding of 2.530x10 moles of this isotope. all particles in the nucleus are shown.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 19:00, MikeWrice3615
How long does it take light from the nearest star other than the sun to reach earth? a) less than 1 second b) about 1 hour c) about 1 month d) about 4 years
Answers: 1
You know the right answer?
50 points! the isotope shown has a mass of 14.003241 amu. calculate how much energy is released fro...
Questions in other subjects:


Mathematics, 09.11.2020 21:40

Advanced Placement (AP), 09.11.2020 21:40

Mathematics, 09.11.2020 21:40

Mathematics, 09.11.2020 21:40

Mathematics, 09.11.2020 21:40



Mathematics, 09.11.2020 21:40

Mathematics, 09.11.2020 21:40