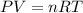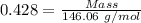Chemistry, 07.12.2019 07:31 lamafiablanca1ovtpyl
Sulfur hexafluoride gas is collected at 26.0 °c in an evacuated flask with a measured volume of 35.0 l. when all the gas has been collected, the pressure in the flask is measured to be 0.300 atm . calculate the mass and number of moles of sulfur hexafluoride gas that were collected. be sure your answer has the correct number of significant digits. mass: s mole

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2

You know the right answer?
Sulfur hexafluoride gas is collected at 26.0 °c in an evacuated flask with a measured volume of 35.0...
Questions in other subjects:


Mathematics, 05.12.2021 05:30

Mathematics, 05.12.2021 05:30



History, 05.12.2021 05:40

Mathematics, 05.12.2021 05:40

Mathematics, 05.12.2021 05:40


Mathematics, 05.12.2021 05:40