
Chemistry, 07.12.2019 04:31 maxy7347go
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is added to 225 ml of 0.00025 m agno3. ksp ag2cro4 =1.1 x 10-12 ii. 0.100l of 0.0015 m mgcl2 and 0.200l of 0.012 m naf. ksp mgf2 = 3.7 x 10-8

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is...
Questions in other subjects:

Social Studies, 19.01.2020 03:31


Mathematics, 19.01.2020 03:31


Advanced Placement (AP), 19.01.2020 03:31





Biology, 19.01.2020 03:31

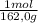 = 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.
= 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.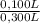 = 0,0005M MgCl₂≡ Mg²⁺
= 0,0005M MgCl₂≡ Mg²⁺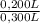 = 0,008M NaF ≡ F⁻
= 0,008M NaF ≡ F⁻


