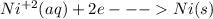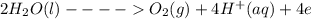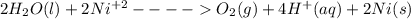Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
Under standard-state conditions, which of the following half-reactions occurs at the cathode during...
Questions in other subjects:




Medicine, 23.02.2021 19:00


Mathematics, 23.02.2021 19:00


Mathematics, 23.02.2021 19:00