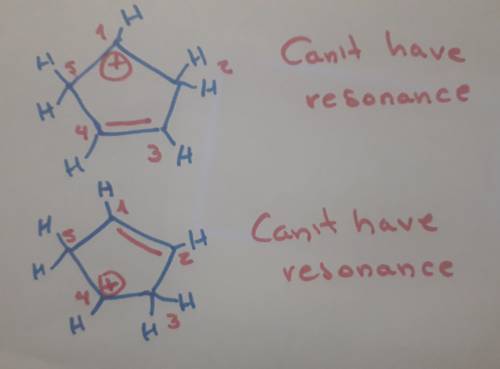
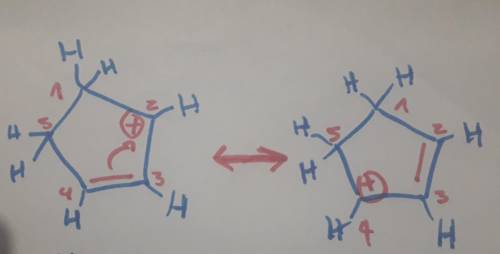
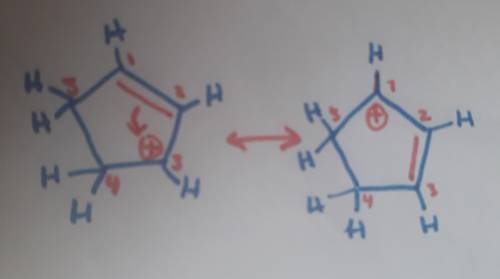
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation. likewise, carbons 2 and 3 are equivalent. write the structure of the carbocation formed by protonation of c−2 or c−3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of c−1 or c−4.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation....
Questions in other subjects:



Mathematics, 05.02.2021 20:50



Mathematics, 05.02.2021 20:50

Mathematics, 05.02.2021 20:50