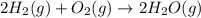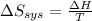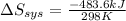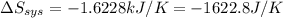Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6...

Chemistry, 07.12.2019 02:31 helloitschump0vfdz
Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6 kj
calculate the following quantities.
delta s sys=
delta s surr=
delta s univ=

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

You know the right answer?
Questions in other subjects:


Mathematics, 19.09.2020 01:01



English, 19.09.2020 01:01

Mathematics, 19.09.2020 01:01

German, 19.09.2020 01:01



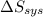 = -1622.8 J/K
= -1622.8 J/K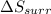 = -94.6 J/K
= -94.6 J/K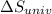 = 0 J/K
= 0 J/K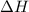 = -483.6 kJ
= -483.6 kJ