
Chemistry, 07.12.2019 02:31 jdvazquez18p7a7vs
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bridge.• a fe electrode in 1.0 m fecl2 solution• a sn electrode in 1.0 m sn(no3)2 solutionwhen the cell is running spontaneously, which choice includes only true statements and no false ones? question 1 options: a. the tin electrode loses mass and the tin electrode is the cathode. b. the tin electrode gains mass and the tin electrode is the cathode. c. the iron electrode gains mass and the iron electrode is the anode. d. the iron electrode loses mass and the iron electrode is the cathode.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3



Chemistry, 22.06.2019 08:30, masontdavis
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bri...
Questions in other subjects:

Mathematics, 26.04.2021 21:00

Chemistry, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00



Spanish, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00

History, 26.04.2021 21:00

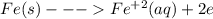
 is higher than tin(ii) ion,
is higher than tin(ii) ion, 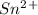 in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a
in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a 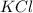 salt bridge:So, an Fe electrode in 1.0 M
salt bridge:So, an Fe electrode in 1.0 M 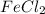 solution and a
solution and a  electrode in 1.0 M
electrode in 1.0 M  solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The
solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The 

