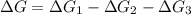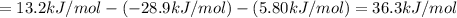Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g =...

Chemistry, 07.12.2019 02:31 queenkimm26
Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g = 13.2 kj/mol
{\rm b \rightleftharpoons c}, \quad\delta g = -28.9 kj/mol
{\rm c \rightleftharpoons d},\quad \delta g = 5.80 kj/mol
what is the free energy, delta g, for the overall reaction, \rm a \rightleftharpoons d ?
express your answer numerically inkilojoules per mole.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 01.03.2021 21:20


Mathematics, 01.03.2021 21:20


English, 01.03.2021 21:20


Physics, 01.03.2021 21:20


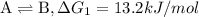 ...[1]
...[1]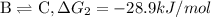 ...[2]
...[2]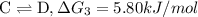 ...[3]
...[3]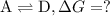 ...[4]
...[4]