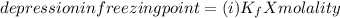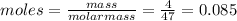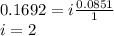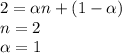Chemistry, 07.12.2019 02:31 kenzieeee96
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a solution is prepared that contains 4.000 g of hno2 in 1.000 kg of water. its freezing point is found to be -0.1692 ∘c calculate the fraction of hno2 that has dissociated.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
When hno2 is dissolved in water it partially dissociates according to the equation hno2⇌h++no−2. a s...
Questions in other subjects:


Mathematics, 17.04.2022 21:40

Mathematics, 17.04.2022 21:40

History, 17.04.2022 21:40


Spanish, 17.04.2022 22:00