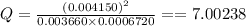Chemistry, 07.12.2019 02:31 aprilkenedy12
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g ) ↽ − − ⇀ 2 brcl ( g ) k c = 7.0 if the composition of the reaction mixture at 400 k is [brcl]=0.004150 [ brcl ] = 0.004150 m, [br2]=0.003660 [ br 2 ] = 0.003660 m, and [cl2]=0.0006720 [ cl 2 ] = 0.0006720 m, what is the reaction quotient, q ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g )...
Questions in other subjects:

History, 15.01.2021 18:50


History, 15.01.2021 18:50


Computers and Technology, 15.01.2021 18:50

English, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50

Mathematics, 15.01.2021 18:50


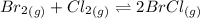
![Q=\frac{[BrCl]^2}{[Br_2][Cl_2]}](/tpl/images/0407/4151/a2d2e.png)
![[BrCl]=0.004150\ M](/tpl/images/0407/4151/33a25.png)
![[Br_2]=0.003660\ M](/tpl/images/0407/4151/93561.png)
![[Cl_2}]=0.0006720\ M](/tpl/images/0407/4151/b53a5.png)