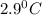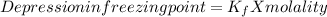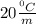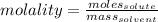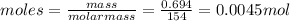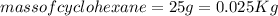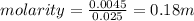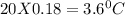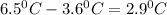Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Cyclohexane has a freezing point of 6.50 ∘c and a kf of 20.0 ∘c/m. what is the freezing point of a s...
Questions in other subjects:





Mathematics, 29.11.2019 07:31