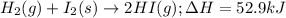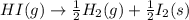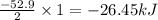Chemistry, 07.12.2019 00:31 hannahs1313
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enthalpy for the following reaction: hi(g) → 1 2h2(g)+1 2 i2(s)? express your answer in kj.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

You know the right answer?
Given the following reaction: h2(g)+i2(s) → 2hi(g) with a ∆h of 52.9 kj. what is the change in enth...
Questions in other subjects:


Mathematics, 19.05.2020 16:04



Mathematics, 19.05.2020 16:04


Mathematics, 19.05.2020 16:04