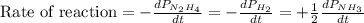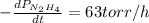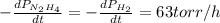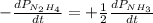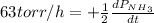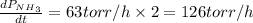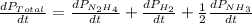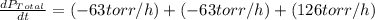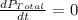Chemistry, 07.12.2019 00:31 kaylarenee05080
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (g) + h2 (g) \rightarrow 2 nh3 (g) is63 torr/h. what are the rates of change of nh3 partial pressure and total pressure in the vessel.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
You know the right answer?
The rate of decrease in n2h4 partial pressure in a closed reaction vessel form the reaction: n2h4 (...
Questions in other subjects:



English, 02.02.2021 23:40

Mathematics, 02.02.2021 23:40


Mathematics, 02.02.2021 23:40



Mathematics, 02.02.2021 23:40

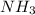 partial pressure is 126 torr/h.
partial pressure is 126 torr/h.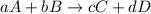
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0407/2122/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0407/2122/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0407/2122/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0407/2122/d4b94.png)
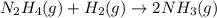
![\text{Rate of disappearance of }N_2H_4=-\frac{d[N_2H_4]}{dt}](/tpl/images/0407/2122/4b6d3.png)
![\text{Rate of disappearance of }H_2=-\frac{d[H_2]}{dt}](/tpl/images/0407/2122/53b46.png)
![\text{Rate of formation of }NH_3=+\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0407/2122/f55ec.png)