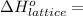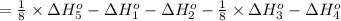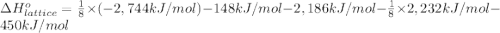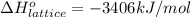Calculate the lattice energy of magnesium sulfide from the data given below. mg(s) → mg(g) δh° = 148 kj/mol mg(g) → mg2+(g) + 2e– δh° = 2186 kj/mol s8(s) → 8s(g) δh° = 2232 kj/mol s(g) + 2e– → s2–(g) δh° = 450 kj/mol 8mg(s) + s8(s) → 8mgs(s) δh° = –2744 kj/mol mgs(s)→mg2+(g) + s2–(g) δh°lattice = ?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
Calculate the lattice energy of magnesium sulfide from the data given below. mg(s) → mg(g) δh° = 148...
Questions in other subjects:










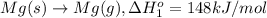 ..[1]
..[1]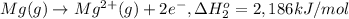 ..[2]
..[2]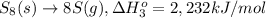 ..[3]
..[3]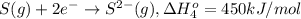 ..[4]
..[4]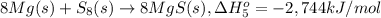 ..[5]
..[5]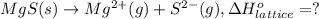 ..[6]
..[6]![[6]=\frac{1}{8}\times [5]-[1]-[2]-\frac{1}{8}\times [3]-[4]](/tpl/images/0407/1216/be747.png)