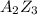Chemistry, 06.12.2019 21:31 bethzabezarahi
Suppose an ionic solid with a cubic unit cell contains a (cations) and z (anions). the a ions are in the body center position and at each corner, and the z ions are on each face. based on this structure the empirical formula of the compound would be az

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
Suppose an ionic solid with a cubic unit cell contains a (cations) and z (anions). the a ions are in...
Questions in other subjects:


English, 11.03.2021 01:00

Mathematics, 11.03.2021 01:00

Social Studies, 11.03.2021 01:00






Mathematics, 11.03.2021 01:00