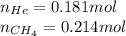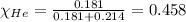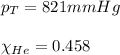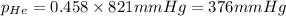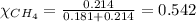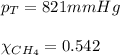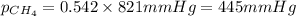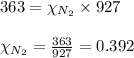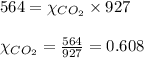Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 09:30, crawford184232323234
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions in other subjects:


Mathematics, 16.07.2021 19:40

Mathematics, 16.07.2021 19:40

Advanced Placement (AP), 16.07.2021 19:40

Mathematics, 16.07.2021 19:40

Mathematics, 16.07.2021 19:40



Mathematics, 16.07.2021 19:40

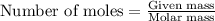 .....(1)
.....(1)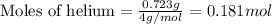
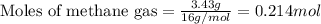
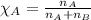 .......(2)
.......(2)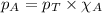 ......(3)
......(3)