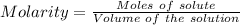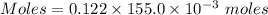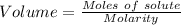Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(a...

Chemistry, 06.12.2019 20:31 milkshakegrande101
Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(aq)+pb(no3)2(aq)→2kno3(aq)+pbi2 (s)
what minimum volume of 0.200 m potassium iodide solution is required to completely precipitate all of the lead in 155.0 ml of a 0.122 m lead(ii) nitrate solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 03.05.2021 15:40

Mathematics, 03.05.2021 15:40



Mathematics, 03.05.2021 15:40


English, 03.05.2021 15:40

Mathematics, 03.05.2021 15:40

Chemistry, 03.05.2021 15:40