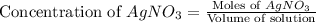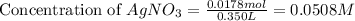Chemistry, 06.12.2019 19:31 orlando19882000
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silver nitrate. calculate the final molarity of iodide anion in the solution. you can assume the volume of the solution doesn't change when the potassium iodide is dissolved in it. be sure your answer has the correct number of significant digits

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silve...
Questions in other subjects:

Business, 10.11.2019 01:31

Mathematics, 10.11.2019 01:31

English, 10.11.2019 01:31





Mathematics, 10.11.2019 01:31

Chemistry, 10.11.2019 01:31

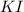 and
and 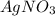 .
.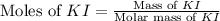
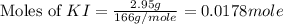
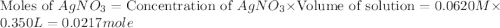
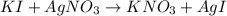
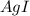
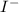 anion = Moles of
anion = Moles of 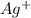 cation = 0.0178 moles
cation = 0.0178 moles