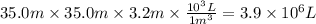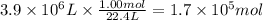Chemistry, 06.12.2019 17:31 keshewar2671
Assuming that all the energy given off in the reaction goes to heating up only the air in the house, determine the mass of methane required to heat the air in a house by 10.0 ∘c. assume each of the following: house dimensions are 35.0 m × 35.0 m × 3.2 m ; specific heat capacity of air is 30 j/k⋅mol; 1.00 mol of air occupies 22.4l for all temperatures concerned.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Assuming that all the energy given off in the reaction goes to heating up only the air in the house,...
Questions in other subjects:



Business, 12.02.2020 02:25

Business, 12.02.2020 02:25