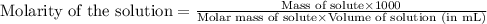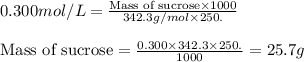Chemistry, 06.12.2019 17:31 kayleighanne3462
You need to prepare 250. ml of a 0.300 m aqueous solution of sucrose, c12h22o11 (aq), which is used frequently in biological experiments. based on your answer above, what is the value of x?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
You need to prepare 250. ml of a 0.300 m aqueous solution of sucrose, c12h22o11 (aq), which is used...
Questions in other subjects:

Mathematics, 01.09.2020 18:01

Social Studies, 01.09.2020 18:01

Social Studies, 01.09.2020 18:01



Computers and Technology, 01.09.2020 18:01


Mathematics, 01.09.2020 18:01


Mathematics, 01.09.2020 18:01

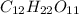 (aq), which is used frequently in biological experiments. Based on your answer above, measure out the amount of solid sucrose in the solution.
(aq), which is used frequently in biological experiments. Based on your answer above, measure out the amount of solid sucrose in the solution.