Calculate either [ h 3 o + ] or [ oh − ] for each of the solutions.
solution a: [ oh − ] = 3...

Calculate either [ h 3 o + ] or [ oh − ] for each of the solutions.
solution a: [ oh − ] = 3.33 × 10 − 7 m
solution a: [ h 3 o + ] = m
solution b: [ h 3 o + ] = 9.33 × 10 − 9 m
solution b: [ oh − ] = m
solution c: [ h 3 o + ] = 5.65 × 10 − 4 m
solution c: [ oh − ] = m

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 06.07.2019 09:30

English, 06.07.2019 09:30



Mathematics, 06.07.2019 09:30





![[H_3O^+]=0.300\times 10^{-7}M](/tpl/images/0406/1496/33e7f.png)
![[OH^-]=0.107\times 10^{-5}M](/tpl/images/0406/1496/20837.png)
![[OH^-]=0.177\times 10^{-10}M](/tpl/images/0406/1496/6aca3.png)
![pH=-\log[H_3O^+]](/tpl/images/0406/1496/a23b5.png)
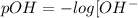
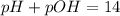
![[H_3O^+][OH^-]=10^{-14}](/tpl/images/0406/1496/f5c30.png)
![[OH^-]=3.33\times 10^{-7}M](/tpl/images/0406/1496/cdc14.png)
![[H_3O^+]=\frac{10^{-14}}{3.33\times 10^{-7}}=0.300\times 10^{-7}M](/tpl/images/0406/1496/59499.png)
![[H_3O^+]=9.33\times 10^{-9}M](/tpl/images/0406/1496/2ba92.png)
![[OH^-]=\frac{10^{-14}}{9.33\times 10^{-9}}=0.107\times 10^{-5}M](/tpl/images/0406/1496/8c201.png)
![[H_3O^+]=5.65\times 10^{-4}M](/tpl/images/0406/1496/ebcc8.png)
![[OH^-]=\frac{10^{-14}}{5.65\times 10^{-4}}=0.177\times 10^{-10}M](/tpl/images/0406/1496/f1735.png)



