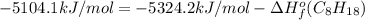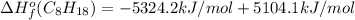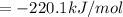Chemistry, 06.12.2019 05:31 breannamiller0822
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of c 8 h 18 ( g ) consumed, under standard conditions. c 8 h 18 ( g ) + 25 2 o 2 ( g ) ⟶ 8 co 2 ( g ) + 9 h 2 o ( g ) δ h ∘ rxn = − 5104.1 kj / mol what is the standard enthalpy of formation of this isomer of c 8 h 18 ( g ) ?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of...
Questions in other subjects:




English, 18.11.2020 18:20

Mathematics, 18.11.2020 18:20

Mathematics, 18.11.2020 18:20

History, 18.11.2020 18:20



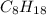 is -220.1 kJ/mol.
is -220.1 kJ/mol.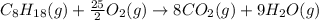
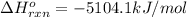
![\Delta H^{o}_{rxn}=[8\Delta H^{o}_{f}(CO_{2}) +9\Delta H^{o}_{f}(H_{2}O)]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}\Delta H^{o}_{f}(O_{2})]](/tpl/images/0405/9197/1d3a2.png)
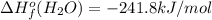
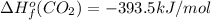
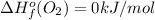
![-5104.1kJ/mol=[8(-393.5)+9(-241.8)kJ/mol]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}(0)kJ/mol]](/tpl/images/0405/9197/edc02.png)