
Chemistry, 06.12.2019 05:31 haitiindianari
Consider the reaction 4 ph3(g) → p4(g) + 6 h2(g). if, in a certain experiment, over a specific time period, 0.0049 mole of ph3 is consumed in a 2.4-l container during each second of the reaction, what are the rates of production of p4 and h2 in this experiment?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, jescanarias22
What’s the scientific notation for the number 6,840,000,000
Answers: 1


Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
Consider the reaction 4 ph3(g) → p4(g) + 6 h2(g). if, in a certain experiment, over a specific time...
Questions in other subjects:




Mathematics, 23.09.2020 06:01





Mathematics, 23.09.2020 06:01

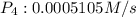
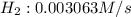
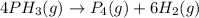
![R=-\frac{1}{4}\frac{d[PH_3]}{dt}=\frac{1}{1}\frac{d[P_4]}{dt}=\frac{1}{6}\frac{d[H_2]}{dt}](/tpl/images/0405/9326/1b2ce.png)
![-\frac{d[PH_3]}{dt}](/tpl/images/0405/9326/0026c.png)
![\frac{[PH_3]}{1 s}](/tpl/images/0405/9326/00de6.png)
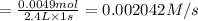
![-\frac{d[PH_3]}{dt}=0.002042 M/s](/tpl/images/0405/9326/a6182.png)
![R = -\frac{1}{4}\frac{d[PH_3]}{dt}=\frac{1}{4}\times 0.002042 M/s=0.0005105 M/s](/tpl/images/0405/9326/7d03f.png)
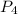 :
:![R=\frac{1}{1}\frac{d[P_4]}{dt}](/tpl/images/0405/9326/34938.png)
![\frac{d[P_4]}{dt}=\frac{1}{1}\times 0.0005105 M/s=0.0005105 M/s](/tpl/images/0405/9326/4315e.png)
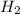 :
:![R=\frac{1}{6}\frac{d[P_4]}{dt}](/tpl/images/0405/9326/3100b.png)
![\frac{d[H_2]}{dt}=\frac{6}{1}\times 0.0005105 M/s=0.003063 M/s](/tpl/images/0405/9326/b8130.png)


