
Chemistry, 06.12.2019 05:31 AaronMicrosoft15
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the corresponding δg∘ and e∘cel under standard conditions.
δg∘ =
e∘cell =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

You know the right answer?
If the equilibrium constant for a two-electron redox reaction at 298 k is 1.0×10−4, calculate the co...
Questions in other subjects:


Mathematics, 21.07.2021 17:10

Mathematics, 21.07.2021 17:10

Mathematics, 21.07.2021 17:10


English, 21.07.2021 17:10

Mathematics, 21.07.2021 17:10

Social Studies, 21.07.2021 17:10

Mathematics, 21.07.2021 17:10


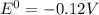

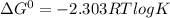



 = gibbs free energy = 22823J
= gibbs free energy = 22823J = standard emf
= standard emf



