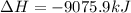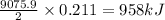Chemistry, 06.12.2019 05:31 lindseylewis313
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released when 0.211 mol of b5h9 reacts with excess oxygen where the products are b2h3(s) and h2o(l). the standard enthalpy of formation of b5h9(l) is 73.2 kj/mol, the standard enthalpy of formation of b2h3(s) is -1272 kj/mol and that of h2o(l) is -285.4 kj/mol. express your answer in kj.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2


You know the right answer?
B5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released wh...
Questions in other subjects:

Health, 08.12.2019 22:31



Mathematics, 08.12.2019 22:31


Mathematics, 08.12.2019 22:31

Health, 08.12.2019 22:31


Chemistry, 08.12.2019 22:31

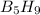 reacts with excess oxygen.
reacts with excess oxygen.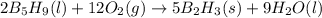
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0405/9477/76c37.png)
![\Delta H=[(n_{B_2H_3}\times \Delta H_{B_2H_3})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{B_5H_9}\times \Delta H_{B_5H_9})]](/tpl/images/0405/9477/bc69c.png)
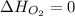 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![\Delta H=[(5\times -1272)+(9\times -285.5]-[(12\times 0)+(2\times 73.2)]](/tpl/images/0405/9477/56397.png)