
Chemistry, 06.12.2019 05:31 smkw04p3ao0n
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hcl(g) calculate the equilibrium concentration of hcl when 0.564 moles of nh4cl(s) are introduced into a 1.00 l vessel at 548 k.[hcl] = m

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hc...
Questions in other subjects:

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50


Mathematics, 12.02.2021 22:50

Arts, 12.02.2021 22:50

English, 12.02.2021 22:50


Mathematics, 12.02.2021 22:50

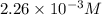
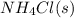 = 0.564 moles
= 0.564 moles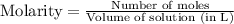
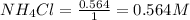
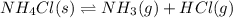
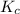 for above equation follows:
for above equation follows:![K_c=[NH_3][HCl]](/tpl/images/0405/9498/72be1.png)
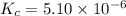
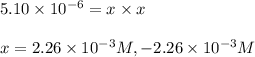
![[HCl]=2.26\times 10^{-3}M](/tpl/images/0405/9498/283fd.png)


