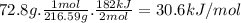Chemistry, 06.12.2019 04:31 phillipsk5480
Achemist measures the energy change ? h during the following reaction: 2hgo (s) ? 2hg (l) +o2 (g) =? h182.kj use the information to answer the following questions. this reaction
a. endothermic.
b. exothermic.
suppose 72.8g of hgo react. will any heat be released or absorbed?
a. yes, absorbed.
b. yes, released.
c. no.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Achemist measures the energy change ? h during the following reaction: 2hgo (s) ? 2hg (l) +o2 (g) =...
Questions in other subjects:

History, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00




Mathematics, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00


Mathematics, 26.04.2021 01:00

Mathematics, 26.04.2021 01:00