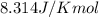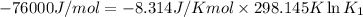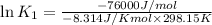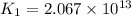Chemistry, 06.12.2019 04:31 emilybomar7466
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15k.
delta g (kj/mol)
hcl=-95.3
o2=0
h2o=-228.6
cl2=0

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

You know the right answer?
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in...
Questions in other subjects:

Mathematics, 21.12.2019 13:31



Social Studies, 21.12.2019 13:31




Mathematics, 21.12.2019 13:31


Biology, 21.12.2019 13:31

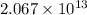 .
.![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0405/8552/b00b4.png)
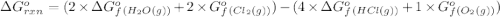
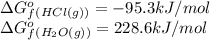
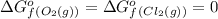 (pure element)
(pure element)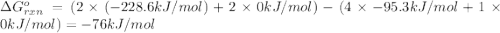
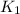 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: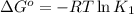
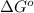 = Gibbs free energy = -76 kJ/mol = -76000 J/mol
= Gibbs free energy = -76 kJ/mol = -76000 J/mol