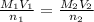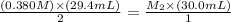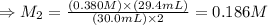Chemistry, 06.12.2019 02:31 NathanFrase6770
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a known concentration of s 2 o 2 − 3 ( aq ) (thiosulfate ion). the determination is based on the net ionic equation 2 s 2 o 2 − 3 ( aq ) + i − 3 ( aq ) ⟶ s 4 o 2 − 6 ( aq ) + 3 i − ( aq ) given that it requires 29.4 ml of 0.380 m na 2 s 2 o 3 ( aq ) to titrate a 30.0 ml sample of i − 3 ( aq ) , calculate the molarity of i − 3 ( aq ) in the solution.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, netflixacc0107
Achemist requires 6.00 liters of 0.320 m h2so4 solution. how many grams of h2so4 should the chemist dissolve in water? 121 grams 159 grams 176 grams 188 grams
Answers: 2

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1
You know the right answer?
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a...
Questions in other subjects:


Mathematics, 14.10.2020 22:01

Mathematics, 14.10.2020 22:01

Mathematics, 14.10.2020 22:01


Mathematics, 14.10.2020 22:01


History, 14.10.2020 22:01

English, 14.10.2020 22:01

Mathematics, 14.10.2020 22:01