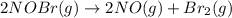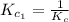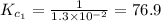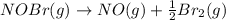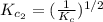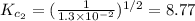Chemistry, 06.12.2019 02:31 taylorb9893
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at this temperature does the equilibrium favor no and br2, or does it favor nobr? calculate kc for 2nobr(g)⥫⥬==2no(g)+br2(g). calculate kc for nobr(g)⥫⥬==no(g)+12br2(g).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 16:00, Karinaccccc
The electron configuration for chromium is 1s22s22p63s23p63d54s1 instead of 1s22s22p63s23p63d44s1. the configuration is an exception to the pauli exclusion principle heisenberg uncertainty principle aufbau principle schrödinger equation
Answers: 3

Chemistry, 23.06.2019 17:30, lyne29
Which of the following elements would you expect to have the highest ionization energy value, and why? a. chlorine (cl), because it has a low effective nuclear charge and large radius b. fluorine (f), because it has a large radius and naturally forms a negative ion c. lithium (li), because it has a small radius and naturally forms a positive ion d. neon (ne), because it has a high effective nuclear charge and small radius
Answers: 2
You know the right answer?
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at thi...
Questions in other subjects:

Advanced Placement (AP), 02.05.2021 01:40





Social Studies, 02.05.2021 01:40




Chemistry, 02.05.2021 01:40

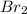 .
.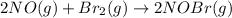
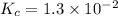 .
.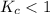 that means equilibrium lies to the left side. Thus, the equilibrium favors NO and
that means equilibrium lies to the left side. Thus, the equilibrium favors NO and