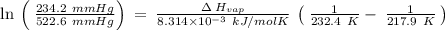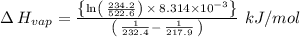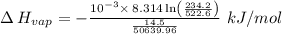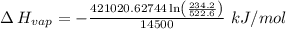Chemistry, 06.12.2019 00:31 graceaowen
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pressure data are obtained.
calculate the enthalpy of vaporization (? hvap) in kj/mol for this liquid.
p1 = 234.2 mmhg t1 = 217.9 k
p2 = 522.6 mmhg t2 = 232.4 k

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pres...
Questions in other subjects:


Mathematics, 26.10.2021 21:50


Social Studies, 26.10.2021 21:50

Mathematics, 26.10.2021 21:50



English, 26.10.2021 21:50


Mathematics, 26.10.2021 21:50

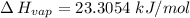
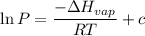
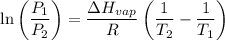
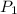 = 234.2 mmHg
= 234.2 mmHg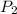 = 522.6 mmHg
= 522.6 mmHg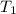 = 217.9 K
= 217.9 K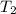 = 232.4 K
= 232.4 K