
Chemistry, 05.12.2019 23:31 maljoh8249
Enter your answer in the provided box. an industrial chemist studying bleaching and sterilizing prepares a hypochlorite buffer using 0.450 m hclo and 0.450 m naclo. (ka for hclo = 2.9 × 10−8) find the ph of 1.00 l of the solution after 0.040 mol of naoh has been added.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3
You know the right answer?
Enter your answer in the provided box. an industrial chemist studying bleaching and sterilizing prep...
Questions in other subjects:


Mathematics, 01.02.2021 20:50

Mathematics, 01.02.2021 20:50


Chemistry, 01.02.2021 20:50

Mathematics, 01.02.2021 20:50





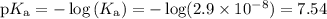
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 7.54 +\log \left(\dfrac{0.450}{0.450}\right )\\\\& = & 7.54 + \log1.00 \\ & = & 7.54 + 0.00\\& = & 7.54\\\end{array}](/tpl/images/0405/3467/7ad02.png)

![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 7.54 +\log \left(\dfrac{490}{410}\right )\\\\& = & 7.54 + \log1.195 \\& = & 7.54 +0.0774\\& = & \mathbf{7.62}\\\end{array}\\\text{The new pH is $\large \boxed{\textbf{7.62}}$}](/tpl/images/0405/3467/ab2c6.png)



