
Chemistry, 05.12.2019 22:31 cuthbertson157
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of carbon dioxide at 20.0 â°c and 791 mmhg. calculate the percent by mass of calcium carbonate in the sample.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

You know the right answer?
Dissolving 5.28 g of an impure sample of calcium carbonate in hydrochloric acid produced 1.14 l of c...
Questions in other subjects:



Biology, 05.05.2020 17:26


Mathematics, 05.05.2020 17:26

Mathematics, 05.05.2020 17:26

Health, 05.05.2020 17:26

Social Studies, 05.05.2020 17:26

Mathematics, 05.05.2020 17:26

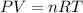
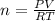

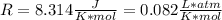
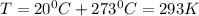

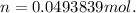
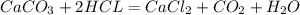
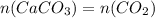
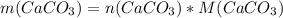
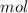 * 100
* 100 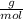 = 4.93839
= 4.93839 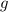
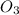 =
= 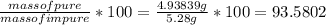 .
.

