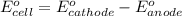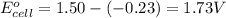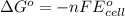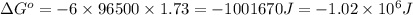Chemistry, 05.12.2019 22:31 angel0203wilcox
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of standard reduction potentials. 2 au 3 + (aq) + 3 ni (s) − ⇀ ↽ − 2 au (s) + 3 ni 2 + (aq) 2au3+(aq)+3ni(s)↽−−⇀2au(s)+3ni2+(aq ) δ g ∘ = δg∘=

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of st...
Questions in other subjects:


Mathematics, 01.09.2019 19:50


Mathematics, 01.09.2019 19:50



Mathematics, 01.09.2019 19:50

History, 01.09.2019 19:50


Health, 01.09.2019 19:50

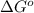 for the given reaction is
for the given reaction is 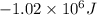
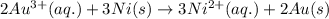
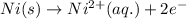 ( × 3)
( × 3)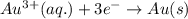 ( × 2)
( × 2)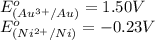
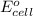 of the reaction, we use the equation:
of the reaction, we use the equation: