
Chemistry, 05.12.2019 22:31 nana54muller
You have 49.8 g of o2 gas in a container with twice the volume as one with co2 gas. the pressure and temperature of both containers are the same. calculate the mass of carbon dioxide gas you have in the container.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2


Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
You have 49.8 g of o2 gas in a container with twice the volume as one with co2 gas. the pressure and...
Questions in other subjects:



Mathematics, 04.12.2020 19:20

Mathematics, 04.12.2020 19:20

Chemistry, 04.12.2020 19:20

English, 04.12.2020 19:20



Mathematics, 04.12.2020 19:20

Physics, 04.12.2020 19:20

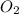 :-
:-
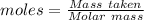
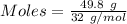
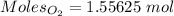
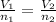
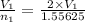
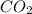 = 44.0 g/mol
= 44.0 g/mol


