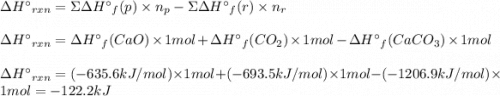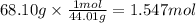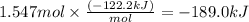Chemistry, 05.12.2019 22:31 20stirltrer
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f values of the reactant and products are the same at 850°c as they are at 25°c, calculate the enthalpy change (in kj) if 68.10 g of co2 is produced in one reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1

Chemistry, 23.06.2019 05:30, victoria6929
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
At 850°c, caco3 undergoes substantial decomposition to yield cao and co2. assuming that the δh o f v...
Questions in other subjects:

Mathematics, 09.11.2019 20:31

Mathematics, 09.11.2019 20:31

Biology, 09.11.2019 20:31






Geography, 09.11.2019 20:31