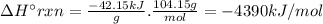Chemistry, 05.12.2019 20:31 leeamation31
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°c, 42.15 kj are released per gram of styrene. find the standard enthalpy of formation of styrene at 25°c.
(given: ? h°f[co2(g)] = –393.5 kj/mol, ? h°f[h2o(l)] = –285.8 kj/mol, ? h°f[h2o(g)] = –241.8 kj/mol)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 13:00, jacobanderson43
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2

Chemistry, 23.06.2019 13:30, jcastronakaya
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
You know the right answer?
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene bur...
Questions in other subjects:

Biology, 17.09.2019 22:30

History, 17.09.2019 22:30

World Languages, 17.09.2019 22:30




History, 17.09.2019 22:30

Mathematics, 17.09.2019 22:30


Health, 17.09.2019 22:30