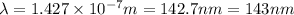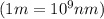Chemistry, 05.12.2019 20:31 arianawelsh123l
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon.
round your answer to 3 significant digits in nm.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
You know the right answer?
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light...
Questions in other subjects:


English, 18.12.2019 20:31

History, 18.12.2019 20:31


Physics, 18.12.2019 20:31

Chemistry, 18.12.2019 20:31



History, 18.12.2019 20:31

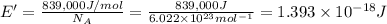
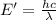 (Using planks equation)
(Using planks equation)