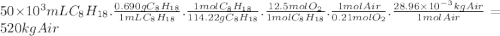Chemistry, 05.12.2019 19:31 mayamcmillan11
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (approximately 13.2 gallons), determine mass of air in kg required to combustion it. you can use isooctane c8h18 to approximate gasoline

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (a...
Questions in other subjects:

History, 27.12.2019 00:31

English, 27.12.2019 00:31

History, 27.12.2019 00:31






Health, 27.12.2019 00:31

Mathematics, 27.12.2019 00:31