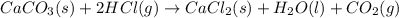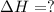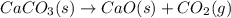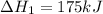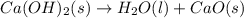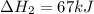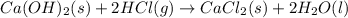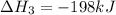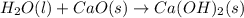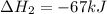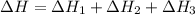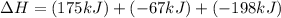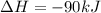Consider the following chemical equations to answer the question that follows. caco3(s)ca(oh)2(s)ca(oh)2(s)+2hcl(g )→cao(s)+co2(g)→h2o(l)+cao(s)→cacl2 (s)+2h2o(l)δhδhδh=175kj=67kj=−198kj using the information above, determine the change in enthalpy for the following chemical reaction. caco3(s)+2hcl(g)⟶cacl2(s)+h2o(l)+co 2(g)

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Consider the following chemical equations to answer the question that follows. caco3(s)ca(oh)2(s)ca(...
Questions in other subjects:


Mathematics, 27.01.2021 01:00



Chemistry, 27.01.2021 01:00

Spanish, 27.01.2021 01:00


English, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00