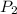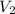Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, gonzalesalexiaouv1bg
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
Afixed amount of gas at 25.0°c occupies a volume of 10.0 l when the pressure is 751 torr. use boyle'...
Questions in other subjects:

History, 14.05.2021 21:50



English, 14.05.2021 21:50




Mathematics, 14.05.2021 21:50


Mathematics, 14.05.2021 21:50

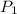
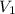 =
=