

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 11:30, starfox5454
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2
You know the right answer?
What is the value for δsºreaction for the following reaction, given the standard entropy values? al...
Questions in other subjects:

Computers and Technology, 28.05.2021 09:50

Chemistry, 28.05.2021 09:50

Spanish, 28.05.2021 09:50


Health, 28.05.2021 09:50



English, 28.05.2021 09:50


Biology, 28.05.2021 09:50

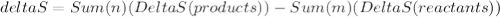 . There are m and n in formula that are coefficients of the reactants and products that are given in the equation.
. There are m and n in formula that are coefficients of the reactants and products that are given in the equation.

