
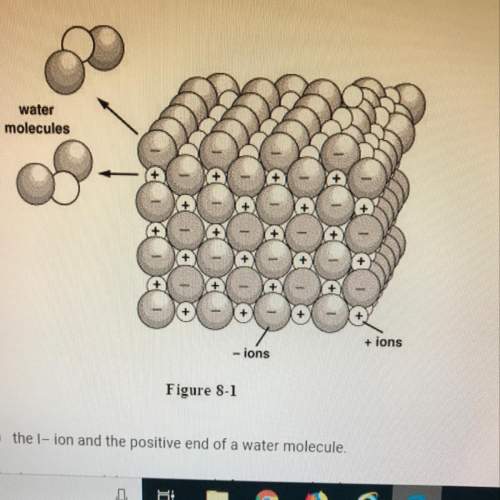
Iwill mark brainliest if the answer is study figure 8-1. when the ionic compound kl is dissolved in water, the k+
ions are pulled into solution by the attraction between
a. the i- ion and the positive end of a water molecule.
b. the k+ ion and the negative end of a water molecule.
c. the k+ and i- ions.
d. the i- ion and the negative end of a water molecule.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Iwill mark brainliest if the answer is study figure 8-1. when the ionic compound kl is dissolved in...
Questions in other subjects:

Mathematics, 10.06.2021 09:30



English, 10.06.2021 09:30

Mathematics, 10.06.2021 09:40

French, 10.06.2021 09:40

Chemistry, 10.06.2021 09:40

Chemistry, 10.06.2021 09:40

English, 10.06.2021 09:40



