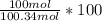Chemistry, 05.12.2019 05:31 goreimani9
Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer. when the flask contents are at 58.3°c, the height of the mercury in the arm of the manometer connected to the flask is 747 mm, and that in the arm open to the atmosphere is 52 mm. at 110°c, the mercury level is 577 mm in the arm connected to the flask, and 222 mm in the other arm. atmospheric pressure is 755 mmhg. (a) using the data given, find δhv and b in the clausius-clapeyron equation. (b) air saturated with chlorobenzene at 130°c and 101.3kpa (absolute) is cooled to 58.3°c at constant pressure. estimate the percentage of the chlorobenzene originally in the vapor that condenses. (hint: draw a flowchart with separate vapor and liquid streams)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer. when the flask...
Questions in other subjects:


History, 04.02.2020 22:51


History, 04.02.2020 22:51



Biology, 04.02.2020 22:51

Biology, 04.02.2020 22:52


Social Studies, 04.02.2020 22:52

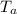 = 58.3°C
= 58.3°C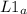 = 747mmHg
= 747mmHg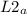 = 52mmHg
= 52mmHg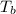 = 110°C
= 110°C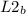 = 222mmHg
= 222mmHg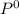 =
= 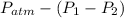
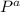 = 755mmHg - (747mmHg - 52mmHg)
= 755mmHg - (747mmHg - 52mmHg)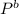 = 755mmHg - (577mmHg = 222mmHg)
= 755mmHg - (577mmHg = 222mmHg)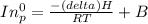
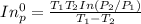
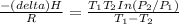
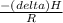 =
= 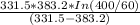
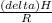 = 4661.4k
= 4661.4k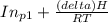
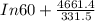
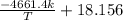
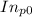 =
= 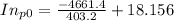
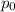 =
= 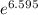
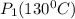 = 731.44mmHg
= 731.44mmHg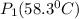 = 60mmHg
= 60mmHg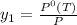
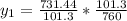
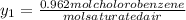

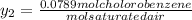
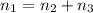
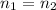 + 100mol
+ 100mol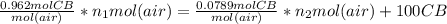
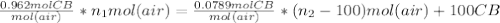
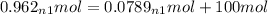
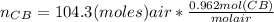
 = 100.34mol CB
= 100.34mol CB