
Chemistry, 05.12.2019 05:31 Damagingawsomeness2
A50.0 ml sample containing cd2+ and mn2+ was treated with 58.4 ml of 0.0400 m edta . titration of the excess unreacted edta required 15.9 ml of 0.0130 m ca2+ . the cd2+ was displaced from edta by the addition of an excess of cn− . titration of the newly freed edta required 22.4 ml of 0.0130 m ca2+ . what are the concentrations of cd2+ and mn2+ in the original solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1


Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
A50.0 ml sample containing cd2+ and mn2+ was treated with 58.4 ml of 0.0400 m edta . titration of th...
Questions in other subjects:


Health, 17.11.2020 18:00



Mathematics, 17.11.2020 18:00



Spanish, 17.11.2020 18:00


Health, 17.11.2020 18:00

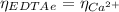
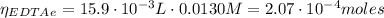
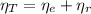 (1)
(1)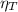 : is the total moles of EDTA,
: is the total moles of EDTA, 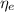 : is the EDTA excess moles and
: is the EDTA excess moles and 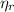 : is the EDTA moles that react with Cd²⁺ and Mn²⁺
: is the EDTA moles that react with Cd²⁺ and Mn²⁺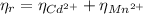 (2)
(2)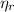 :
: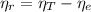
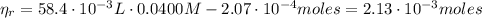
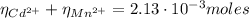 (3)
(3)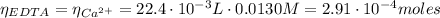
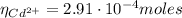
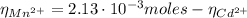
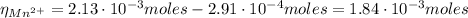
![[Cd^{2+}] = \frac{2.91 \cdot 10^{-4} moles}{50.0\cdot 10^{-3}L} = 5.82 \cdot 10^{-3} M](/tpl/images/0404/1338/54447.png)
![[Mn^{2+}] = \frac{1.84 \cdot 10^{-3} moles}{50.0\cdot 10^{-3}L} = 0.037M](/tpl/images/0404/1338/68079.png)



