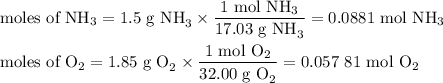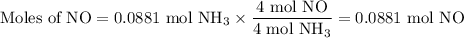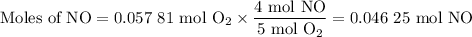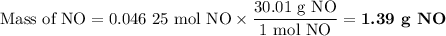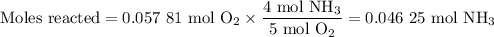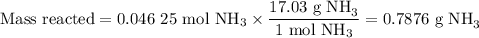no:

Chemistry, 04.12.2019 19:31 ErickP1686
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
nh3 + o2 + no + h2o
balanced: 4nh3 +502 uno+ 6h2o
a.) how many grams of no form when 1.5g of nh3 reacts with 1.85g of o2? b.) which
reactant is the limiting reactant and which one is the excess reactant? c.) how much of
the excess reactant remains after the limiting reactant is completely consumed?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
no:
Questions in other subjects:

Mathematics, 13.01.2020 03:31




Mathematics, 13.01.2020 03:31

Advanced Placement (AP), 13.01.2020 03:31

Mathematics, 13.01.2020 03:31



History, 13.01.2020 03:31