
Chemistry, 04.12.2019 06:31 jaedenevan062907
Which of the following statements explain why the van der waals equation must be used to describe real gases? x. interactions between gas molecules reduces the temperature of the gas in the sample y. the non-zero volumes of gas particles effectively decrease the amount of "empty space" between them z. the molecular attractions between particles of gas decreases the pressure exerted by the gas

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Which of the following statements explain why the van der waals equation must be used to describe re...
Questions in other subjects:

Mathematics, 01.03.2020 05:41

Chemistry, 01.03.2020 05:41

Mathematics, 01.03.2020 05:41

Mathematics, 01.03.2020 05:41

English, 01.03.2020 05:41


Mathematics, 01.03.2020 05:41

Mathematics, 01.03.2020 05:41


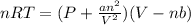 (1)
(1)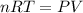 (2)
(2)

