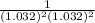Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made of lead and the cathode is made of lead(iv) oxide. both are submerged in 4.30 m sulfuric acid. the half-reactions are: pbo_2(s) + 3h^+ (aq) + hso_4^- (aq) + 2e^- rightarrow pbso_4(s) + 2h_2o(l) e degree = 1.685 v pbso_4(s) + h^+(aq) + 2e^- rightarrow pb(s) + hso_4^- (aq) e degree = -0.356 v (a) calculate the value of e degree. (b) determine the initial value of e_cell. assume that the first ionization of h_2so_4 is complete and that [h^+] almostequalto [hso_4^-]. (c) find e_cell when the h^+ concentration has dropped by 76.00%. again, assume [h^+] almostequalto [hso_4^-].

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made...
Questions in other subjects:

Mathematics, 26.08.2020 09:01



Mathematics, 26.08.2020 09:01



English, 26.08.2020 09:01



Mathematics, 26.08.2020 09:01

 →
→ 
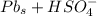 →
→ 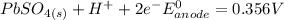
 →
→ 
 →
→ 
 such that (;)
such that (;)  & b=
& b= 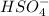
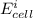 =
=  -
- 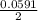 × log
× log 



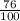
 = 2.041V -
= 2.041V -